THERAPEUTIC INDICATIONS:
-
catarrhal sore throat;
-
acute and chronic tonsillitis, pharyngitis;
-
aphthous stomatitis.
 Download instructions for use
Download instructions for use
Composition
1 g of the product contains:
- active substance - iodine 0.01 g;
- excipients: potassium iodide, 85% glycerin, purified water.
Pharmaceutical form
Spray for external use 1% 30 g and 50 g.
Pharmacotherapeutic group
Medications for throat diseases. Antiseptics. Other drugs.
ATC code R02AА20
Indications
- catarrhal sore throat;
- acute and chronic tonsillitis, pharyngitis;
- aphthous stomatitis.
Contraindications
- individual hypersensitivity to iodine and other components of the product;
- pregnancy and lactation period;
- hyperfunction of the thyroid gland;
- decompensated heart and renal failure;
- diagnostic tests or therapy with radioactive iodine (2 weeks before and after tests or therapy);
- dermatitis herpetiformis;
- children under 8 years of age.
Posology and method of administration
The product is used in adults and children over 8 years of age topically after meals 2-4 times a day.
Remove the bottle from the plastic bag, remove the protective cap from the head and the end of the atomizer tube, set the atomizer tube position up or to the side, gently press its head 3-4 times so that the solution came to the atomizer and after pressing it sprayed (Fig.1)
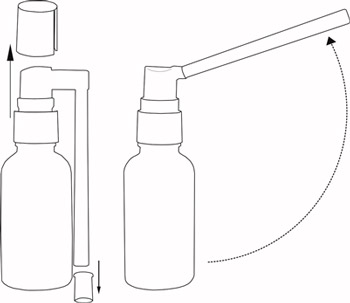
Fig.1
Put the protective caps back on after applying Lugolit-DF®.
If using Lugolit-DF® in a bottle complete with a mechanical atomizer, the cap of the bottle must be replaced with the atomizer before use.
To treat sore throat, tonsillitis or pharyngitis, introduce the atomizer 2-3 cm into the oral cavity, hold your breath and press 2 times on the head of the atomizer so that one irrigation is done to the right, the second to the left.
When treating stomatitis it is necessary to irrigate the oral mucosa.
The duration of use depends on the nature of the disease, its severity and is determined by the doctor.
Apply the product carefully, avoiding contact with the respiratory tract!
Adverse reactions
- locally irritating effect (burning);
- allergic reactions (itching, hyperemia, urticaria);
- iodism (metallic taste, runny nose, urticaria, Quincke's edema, salivation, lacrimation, gastrointestinal distress).
- If side effects occur, it is necessary to stop using the product.
Overdose
No cases of overdose have been observed with the recommended topical application of the product in the oral cavity or pharynx.
Symptoms: If accidentally swallowed, acute poisoning is possible. First, there is a metallic taste in the mouth, possibly vomiting, stomach pain, and liquid stools. Anuria, vocal cleft edema with subsequent development of asphyxia, aspiration pneumonia or pulmonary edema are observed over 1-3 days. In some cases, there may be a circulatory disturbance.
Treatment: Gastric lavage is performed. The patient should take activated charcoal or a 1-5% solution of sodium thiosulfate, and then give milk or brewed starch to drink. Then symptomatic treatment is carried out. There is no specific antidote.
In case of accidental contact with the eyes, they should be rinsed with plenty of water or sodium thiosulfate solution.
Use during pregnancy or breastfeeding
Contraindicated.
Pediatric population
In children over 8 years of age it can cause reflex bronchospasm, so the product should be used on the doctor's recommendation.
Special warnings
The product is not intended for oral administration.
Use with caution in laryngitis due to the possibility of laryngospasm, tuberculosis, hemorrhagic diathesis.
If you are taking other medications, you should consult your doctor about the possibility of using Lugolit-DF®.
Effects on ability to drive and use machines
Given the side effects, caution should be exercised when driving motor vehicles or potentially dangerous machinery
Presentation and package
30 g of the product in dark glass bottles with a screw neck, with a mechanical atomizer and a protective cap on the head and the end of the atomizer tube. The bottle is placed in a plastic bag. One bottle is with instruction for use in the state and Russian language are placed in a carton pack.
Or, 30 g of the product in dark glass bottles with screw necks, sealed with a screwed plastic lid, placed in a plastic bag, complete with a mechanical atomizer fitted with a protective cap on the head and the end of the atomizer tube. One bottle with a mechanical atomizer and instructions for use in the state and Russian languages are placed in a carton pack.
Storage conditions
Store in a place protected from light, at a temperature below 25оС.
Keep out of the reach of children.
Shelf life
3 years
The period of use after opening the bottle complete with atomizer is 28 days.
Manufacturer
"DOSFARM" LLP, 3 Chaplygin street, Almaty city, 050034, Kazakhstan, tel.: (727) 253-03-88.
Marketing authorization holder
"DOSFARM" LLP, 3 Chaplygin street, Almaty city, 050034, Kazakhstan.
Name, address, contact information of the organization accepting claims (proposals) against quality of products from consumers in the territory of the Republic of Kazakhstan, which is responsible for post-authorization safety surveillance of the medicine:
"DOSFARM" LLP, 3 Chaplygin street, Almaty city, 050034, Kazakhstan,
Tel./Fax: (727) 253-07-07, 253-03-88, e-mail: Этот адрес электронной почты защищён от спам-ботов. У вас должен быть включен JavaScript для просмотра.